Mechanisms of Chemopreventive and Therapeutic Proprieties of Ginger Extracts in Cancer
Abstract
:1. Introduction
2. Ginger Derivatives and Cell Cycle Arrest
3. Ginger and Cellular Death
4. Ginger, Its Constituents, and ROS Balance
5. Ginger and Angiogenesis
6. Cancer Stem Cells, Epithelial-Mesenchymal Transition and Ginger
7. Ginger and Multidrug Resistance
8. Ginger Enhanced Bioavailability and Combined Treatment
9. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AP-1 | activator protein 1 |
| AST | aspartate aminotransferase |
| ATPase | adenosine triphosphatase |
| B-ALL | B-cell Acute Lymphoblastic Leukemia |
| Bcl-2 | B-cell lymphoma 2 |
| CAM | chorioallantoic membrane |
| Caspases | cysteine-aspartate proteases |
| CAT | catalase |
| CDK | cyclin-dependent kinases |
| CFEZO | crude flavonoid extracts from Z. officinale |
| CKI | cyclin-dependent kinases inhibitor |
| COX-2 | cyclooxygenase-2 |
| CRC | colorectal carcinoma |
| CSCs | cancer stem cells |
| CuZn-SOD Cu-Zn | superoxide dismutase |
| CYP-P450 | cytochrome P450 |
| DMBA | 0.5% 7,12-dimethylbenz[a]anthracene |
| DNA | deoxyribonucleic acid |
| DNPH | dinitrophenylhydrazone |
| ECOG | Eastern Cooperative Oncology Group |
| EE | Etlingera elatior |
| EMT | epithelial-mesenchymal transition |
| eNO | endothelial nitric oxide synthase |
| ERK | Extracellular signal-regulated kinase |
| FAs | focal adhesions |
| GAE | aqueous extract of ginger |
| GDNVs | ginger-derived nanovectors |
| GR | glutathione reductase |
| GSH | Glutathione |
| GST | glutathione-S-transferase |
| HBP | hamster buccal pouch |
| HNSCC | head and neck squamous cell carcinoma |
| HUVECs | human umbilical vein endothelial cells |
| IAP | inhibitor of apoptosis |
| IL-8 | interleukin 8 |
| LC3-II | light chain3-II |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA | malondialdehyde |
| MDR | multidrug resistance |
| MET | esenchymal–epithelial transition |
| miRs | microRNA |
| MMP | Matrix metalloproteinase |
| MRP1 | multidrug resistance-associated protein 1 |
| mTOR | the mechanistic target of rapamycin |
| MTX | methotrexate |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nox | NADPH oxidases |
| NSCLC | non-small-cell lung cancer |
| PEG | Polyethylene Glycol |
| Pgp | P-glycoprotein |
| PI3K | phosphatidylinositol-3-kinase |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| SCID | severe combined immune deficient |
| SDGE | Steam Distilled Extract of Ginger |
| SMs | 6-shogaol loaded micelles |
| SSHE | extract of Syussai ginger |
| STAT3 | signal transducer and activator of transcription 3 |
| T-ALL | T-cell Acute Lymphoblastic Leukemia |
| TNBC | triple negative breast cancer |
| TNF | tumor necrosis factor |
| TRAIL | TNF-related apoptosis-inducing ligand |
| USP14 | ubiquitin-specific peptidase 14 |
| VEGF | vascular endothelial growth factor |
| XO | xanthine oxidase |
| ZOME | Methanolic extract of Zingiber officinale rhizome |
References
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Causes of Death, Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-J.; Chen, C.; Zhao, Y.; Wang, P.C. Circumventing Tumor Resistance to Chemotherapy by Nanotechnology. Methods Mol. Biol. 2009, 596, 467–488. [Google Scholar] [CrossRef] [Green Version]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Hong, C.S.; Lee, S.W.; Nam, J.H.; Kim, B.J. Effects of ginger and its pungent constituents on transient receptor potential channels. Int. J. Mol. Med. 2016, 38, 1905–1914. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.-K.; Tsai, Y.-H.; Korinek, M.; Hung, P.-H.; El-Shazly, M.; Cheng, Y.-B.; Wu, Y.-C.; Hsieh, T.-J.; Chang, F.-R. 6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2017, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Mansingh, D.P.; OJ, S.; Sali, V.K.; Vasanthi, H.R. [6]-Gingerol-induced cell cycle arrest, reactive oxygen species generation, and disruption of mitochondrial membrane potential are associated with apoptosis in human gastric cancer (AGS) cells. J. Biochem. Mol. Toxicol. 2018, 32, e22206. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-C.; Min, J.-K.; Kim, T.-Y.; Lee, S.-J.; Yang, H.-O.; Han, S.; Kim, Y.-M.; Kwon, Y.-G. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005, 335, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Rhode, J.; Fogoros, S.; Zick, S.; Wahl, H.; Griffith, K.A.; Huang, J.; Liu, J.R. Ginger inhibits cell growth and modulates angiogenic factors in ovarian cancer cells. BMC Complement. Altern. Med. 2007, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Babasheikhali, S.R.; Rahgozar, S.; Mohammadi, M. Ginger extract has anti-leukemia and anti-drug resistant effects on malignant cells. J. Cancer Res. Clin. Oncol. 2019, 145, 1987–1998. [Google Scholar] [CrossRef]
- Angelini, A.; Conti, P.; Ciofani, G.; Cuccurullo, F.; Di Ilio, C. Modulation of multidrug resistance P-glycoprotein activity by antiemetic compounds in human doxorubicin-resistant sarcoma cells (MES-SA/Dx-5): Implications on cancer therapy. J. Boil. Regul. Homeost. Agents 2014, 27, 1029–1037. [Google Scholar]
- Ray, A.; Vasudevan, S.; Sengupta, S. 6-Shogaol Inhibits Breast Cancer Cells and Stem Cell-Like Spheroids by Modulation of Notch Signaling Pathway and Induction of Autophagic Cell Death. PLoS ONE 2015, 10, e0137614. [Google Scholar] [CrossRef]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-kappaB signaling. Cancer Prev. Res. 2014, 7, 627–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annamalai, G.; Kathiresan, S.; Kannappan, N. [6]-Shogaol, a dietary phenolic compound, induces oxidative stress mediated mitochondrial dependant apoptosis through activation of proapoptotic factors in Hep-2 cells. Biomed. Pharmacother. 2016, 82, 226–236. [Google Scholar] [CrossRef]
- Kotowski, U.; Kadletz, L.; Schneider, S.; Foki, E.; Schmid, R.; Seemann, R.; Thurnher, D.; Heiduschka, G. 6-shogaol induces apoptosis and enhances radiosensitivity in head and neck squamous cell carcinoma cell lines. Phytother. Res. 2018, 32, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, S.; Govindhan, A. [6]-Shogaol, a Novel Chemopreventor in 7,12-Dimethylbenz[a]anthracene-induced Hamster Buccal Pouch Carcinogenesis. Phytother. Res. 2016, 30, 646–653. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jeon, Y.; Kim, T.; Lim, W.C.; Ham, J.; Park, Y.N.; Kim, T.-J.; Ko, H. Combined treatment with zingerone and its novel derivative synergistically inhibits TGF-β1 induced epithelial-mesenchymal transition, migration and invasion of human hepatocellular carcinoma cells. Bioorganic Med. Chem. Lett. 2017, 27, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Hariri, M.; Darvishi, L.; Mofid, M.R. Anti-Oxidative and Anti-Inflammatory Effects of Ginger in Health and Physical Activity: Review of Current Evidence. Int. J. Prev. Med. 2013, 4, S36–S42. [Google Scholar]
- Habib, S.H.M.; Makpol, S.; Hamid, N.A.A.; Das, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 2008, 63, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Choi, K.S. Autophagy and cancer. Exp. Mol. Med. 2012, 44, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shoji-Kawata, S.; Sumpter, R.M.; Wei, Y.; Ginet, V.; Zhang, L.; Posner, B.; Tran, K.A.; Green, D.; Xavier, R.J.; et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, 20364–20371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.D.L.; Remião, F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef] [Green Version]
- Wani, N.A.; Zhang, B.; Teng, K.-Y.; Barajas, J.M.; Motiwala, T.; Hu, P.; Yu, L.; Brüschweiler, R.; Ghoshal, K.; Jacob, S.T. Reprograming of Glucose Metabolism by Zerumbone Suppresses Hepatocarcinogenesis. Mol. Cancer Res. 2017, 16, 256–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechner, J.F.; Stoner, G.D. Gingers and Their Purified Components as Cancer Chemopreventative Agents. Molecules 2019, 24, 2859. [Google Scholar] [CrossRef] [Green Version]
- Zwezdaryk, K.J.; Combs, J.A.; Morris, C.A.; Sullivan, D.E. Regulation of Wnt/beta-catenin signaling by herpesviruses. World J. Virol. 2016, 5, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coultas, L.; Strasser, A. The role of the Bcl-2 protein family in cancer. Semin. Cancer Biol. 2003, 13, 115–123. [Google Scholar] [CrossRef]
- Vermeulen, K.; Berneman, Z.N.; Van Bockstaele, D.R. Cell cycle and apoptosis. Cell Prolif. 2003, 36, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Hanson, C.J.; Bootman, M.D.; Distelhorst, C.W.; Wojcikiewicz, R.J.; Roderick, H.L. Bcl-2 suppresses Ca2+ release through inositol 1,4,5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium 2008, 44, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis*. Annu Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrario, A.; Rucker, N.; Wong, S.; Luna, M.; Gomer, C.J. Survivin, a Member of the Inhibitor of Apoptosis Family, Is Induced by Photodynamic Therapy and Is a Target for Improving Treatment Response. Cancer Res. 2007, 67, 4989–4995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Wei, S.; Gan, B.; Peng, X.; Zou, W.; Guan, J.L. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes. Dev. 2011, 25, 1510–1527. [Google Scholar] [CrossRef] [Green Version]
- Krajarng, A.; Chulasiri, M.; Watanapokasin, R. Etlingera elatior Extract promotes cell death in B16 melanoma cells via down-regulation of ERK and Akt signaling pathways. BMC Complementary Altern. Med. Vol. 2017, 17, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.R.; Kim, J.Y.; Kang, Y.J.; Ahn, J.Y.; Kim, J.H.; Kim, B.W.; Choi, H.Y.; Jeong, M.Y.; Cho, S.G. Interplay between PI3K/Akt and MAPK signaling pathwaysin DNA-damaging drug-induced apoptosis. Biochim. Biophys. Acta 2006, 1763, 958–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, M.N.; Mowers, E.E.; Drake, L.E.; Collier, C.; Chen, H.; Zamora, M.; Mui, S.; Macleod, K.F. Autophagy Promotes Focal Adhesion Disassembly and Cell Motility of Metastatic Tumor Cells through the Direct Interaction of Paxillin with LC3. Cell Rep. 2016, 15, 1660–1672. [Google Scholar] [CrossRef] [Green Version]
- Meeran, S.M.; Katiyar, S.K. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front. Biosci. A J. Virtual Libr. 2008, 13, 2191–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, S.; Abidin, S.A.Z.; Murad, N.A.; Makpol, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Ginger extract (Zingiber officinale) triggers apoptosis and G0/G1 cells arrest in HCT 116 and HT 29 colon cancer cell lines. Afr. J. Biochem. Res. 2010, 4, 134–142. [Google Scholar]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Mansour, H.E.A. Ginger extract adjuvant to doxorubicin in mammary carcinoma: Study of some molecular mechanisms. Eur. J. Nutr. 2018, 57, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Elkady, A.I.; Hussein, R.A.E.H.; Abu-Zinadah, O.A. Differential control of growth, apoptotic activity and gene expression in human colon cancer cells by extracts derived from medicinal herbs, Rhazya strictaandZingiber officinaleand their combination. World J. Gastroenterol. 2014, 20, 15275–15288. [Google Scholar] [CrossRef]
- Sehrawat, A.; Arlotti, J.A.; Murakami, A.; Singh, S.V. Zerumbone causes Bax- and Bak-mediated apoptosis in human breast cancer cells and inhibits orthotopic xenograft growth in vivo. Breast Cancer Res. Treat. 2012, 136, 429–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pashaei-Asl, R.; Pashaei-Asl, F.; Gharabaghi, P.M.; Khodadadi, K.; Ebrahimi, M.; Ebrahimie, E.; Pashaiasl, M. The Inhibitory Effect of Ginger Extract on Ovarian Cancer Cell Line; Application of Systems Biology. Adv. Pharm. Bull. 2017, 7, 241–249. [Google Scholar] [CrossRef]
- Choudhury, D.; Das, A.; Bhattacharya, A.; Chakrabarti, G. Aqueous extract of ginger shows antiproliferative activity through disruption of microtubule network of cancer cells. Food Chem. Toxicol. 2010, 48, 2872–2880. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Huang, F.; Zhao, J.; Ding, H.; Cunningham, C.; Coad, J.E.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antitumor effect of beta-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell. Mol. Life Sci. 2005, 62, 881–893. [Google Scholar] [CrossRef]
- Liu, Y.; Whelan, R.J.; Pattnaik, B.R.; Ludwig, K.; Subudhi, E.; Rowland, H.; Claussen, N.; Zucker, N.; Uppal, S.; Kushner, D.M.; et al. Terpenoids from Zingiber officinale (Ginger) Induce Apoptosis in Endometrial Cancer Cells through the Activation of p53. PLoS ONE 2012, 7, e53178. [Google Scholar] [CrossRef] [Green Version]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016, 16, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Muller, P.A.J.; Vousden, K.H. p53 mutations in cancer. Nat. Cell Biol. 2013, 15, 2–8. [Google Scholar] [CrossRef]
- Ansari, J.A.; Ahmad, M.K.; Khan, A.R.; Fatima, N.; Khan, H.J.; Rastogi, N.; Mishra, D.P.; Mahdi, A.A. Anticancer and Antioxidant activity of Zingiber officinale Roscoe rhizome. Indian J. Exp. Boil. 2016, 54, 767–773. [Google Scholar]
- Lum, J.J.; Bauer, D.E.; Kong, M.; Harris, M.H.; Li, C.; Lindsten, T.; Thompson, C.B. Growth Factor Regulation of Autophagy and Cell Survival in the Absence of Apoptosis. Cell 2005, 120, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.; Jeong, J.-K.; Seol, J.-W.; Hur, J.; Eo, S.-K.; Lee, J.-H.; Park, S.-Y. Inhibition of the autophagy flux by gingerol enhances TRAIL-induced tumor cell death. Oncol. Rep. 2015, 33, 2331–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna-Dulcey, L.; Tomasin, R.; Naves, M.A.; Da Silva, J.A.; Cominetti, M.R. Autophagy-dependent apoptosis is triggered by a semi-synthetic [6]-gingerol analogue in triple negative breast cancer cells. Oncotarget 2018, 9, 30787–30804. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Xia, C.; Sun, Z. The Inhibitory Effect of 6-Gingerol on Ubiquitin-Specific Peptidase 14 Enhances Autophagy-Dependent Ferroptosis and Anti-Tumor in vivo and in vitro. Front. Pharmacol. 2020, 11, 598555. [Google Scholar] [CrossRef] [PubMed]
- Karna, P.; Chagani, S.; Gundala, S.R.; Rida, P.C.; Asif, G.; Sharma, V.; Gupta, M.V.; Aneja, R. Benefits of whole ginger extract in prostate cancer. Br. J. Nutr. 2012, 107, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Akimoto, M.; Iizuka, M.; Kanematsu, R.; Yoshida, M.; Takenaga, K. Anticancer Effect of Ginger Extract against Pancreatic Cancer Cells Mainly through Reactive Oxygen Species-Mediated Autotic Cell Death. PLoS ONE 2015, 10, e0126605. [Google Scholar] [CrossRef]
- Grivennikova, V.; Kozlovsky, V.S.; Vinogradov, A.D. Respiratory complex II: ROS production and the kinetics of ubiquinone reduction. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017, 1858, 109–117. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Bachi, A.; Dalle-Donne, I.; Scaloni, A. Redox Proteomics: Chemical Principles, Methodological Approaches and Biological/Biomedical Promises. Chem. Rev. 2013, 113, 596–698. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci 2005, 10, 1881–1896. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.-D.; Zheng, H.-M. Zerumbone inhibits prostate cancer cell viability and induces cell death by non-apoptotic pathway. Bangladesh J. Pharmacol. 2016, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Pezzuto, J.M. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002, 21, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Danwilai, K.; Konmun, J.; Sripanidkulchai, B.-O.; Subongkot, S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: A pilot study. Cancer Manag. Res. 2017, 9, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Bussolino, F.; Mantovani, A.; Persico, G. Molecular mechanisms of blood vessel formation. Trends Biochem. Sci. 1997, 22, 251–256. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35, S224–S243. [Google Scholar] [CrossRef]
- Mukherjee, A.; Madamsetty, V.S.; Paul, M.K.; Mukherjee, S. Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer. Int. J. Mol. Sci. 2020, 21, 455. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.O.; Kundu, J.K.; Shin, Y.K.; Prk, J.H.; Cho, M.H.; Kim, T.Y.; Surh, Y.J. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-κ B in phorbol ester-stimulated mouse skin. Oncogene 2005, 24, 2558–2567. [Google Scholar] [CrossRef] [Green Version]
- Bashir, M.F.; Qadir, M.I. Effect of ginger extract on angiogenesis using CAM assay. Bangladesh J. Pharmacol. 2017, 12, 348. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.C.; Shah, C.; Liu, J.; Pham, J.T.; Zhang, J.G.; Jadus, M.R. Ginger’s (Zingiber officinale Roscoe) inhibition of rat colonic adenocarcinoma cells proliferation and angiogenesis in vitro. Phytother Res. 2009, 23, 640–645. [Google Scholar] [CrossRef]
- Pacifico, F.; Leonardi, A. NF-kappaB in solid tumors. Biochem. Pharm. 2006, 72, 1142–1152. [Google Scholar] [CrossRef]
- Marie-Egyptienne, D.T.; Lohse, I.; Hill, R.P. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: Potential role of hypoxia. Cancer Lett. 2013, 341, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Pilozzi, E.L.D. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Todaro, M.; Francipane, M.G.; Medema, J.P.; Stassi, G. Colon Cancer Stem Cells: Promise of Targeted Therapy. Gastroenterology 2010, 138, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Zeuner, A.; Todaro, M.; Stassi, G.; De Maria, R. Colorectal Cancer Stem Cells: From the Crypt to the Clinic. Cell Stem Cell 2014, 15, 692–705. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, A.; Cancelas, J.A. Cancer stem cells: A stride towards cancer cure? J. Cell. Physiol. 2010, 225, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozovska, Z.; Gabrisova, V.; Kucerova, L. Colon cancer: Cancer stem cells markers, drug resistance and treatment. Biomed. Pharmacother. 2014, 68, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Hollier, B.G.; Evans, K.; Mani, S.A. The Epithelial-to-Mesenchymal Transition and Cancer Stem Cells: A Coalition Against Cancer Therapies. J. Mammary Gland. Biol. Neoplasia 2009, 14, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, B.; Liu, F.; Zhang, M.; Wang, Q.; Liu, Y.; Yao, Y.; Li, D. The epithelial to mesenchymal transition (EMT) and cancer stem cells: Implication for treatment resistance in pancreatic cancer. Mol. Cancer 2017, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Ramírez, A.S.; Díaz-Muñoz, M.; Butanda-Ochoa, A.; Vázquez-Cuevas, F.G. Nucleotides and nucleoside signaling in the regulation of the epithelium to mesenchymal transition (EMT). Purinergic Signal. 2016, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, A.; Tadagavadi, R.K.; Swafford, D.; Manicassamy, S. Modulation of Inflammatory Responses by Wnt/beta-Catenin Signaling in Dendritic Cells: A Novel Immunotherapy Target for Autoimmunity and Cancer. Front. Immunol. 2016, 7, 460. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Zhang, X.; Feng, X.; Fan, X.; Jin, Z. The crosstalk between microRNAs and the Wnt/beta-catenin signaling pathway in cancer. Oncotarget 2017, 8, 14089–14106. [Google Scholar] [CrossRef] [Green Version]
- Kinzler, K.W.; Vogelstein, B. Lessons from Hereditary Colorectal Cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Kim, V.N. MicroRNA biogenesis: Coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005, 6, 376–385. [Google Scholar] [CrossRef]
- Ghahhari, N.M.; Babashah, S. Interplay between microRNAs and WNT/beta-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur. J. Cancer 2015, 51, 1638–1649. [Google Scholar] [CrossRef]
- Dermani, F.K.; Amini, R.; Saidijam, M.; Pourjafar, M.; Saki, S.; Najafi, R. Zerumbone inhibits epithelial-mesenchymal transition and cancer stem cells properties by inhibiting the β-catenin pathway through miR-200c. J. Cell. Physiol. 2018, 233, 9538–9547. [Google Scholar] [CrossRef]
- Martin, A.C.B.; Fuzer, A.M.; Becceneri, A.B.; Da Silva, J.A.; Tomasin, R.; DeNoyer, D.; Kim, S.-H.; McIntyre, K.A.; Pearson, H.B.; Yeo, B.; et al. [10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo. Oncotarget 2017, 8, 72260–72271. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, S.; Chen, Z.S.; Kitazono, M.; Sumizawa, T.; Furukawa, T.; Aikou, T. Mechanisms for resistance to anticancer agents and the reversal of the resistance. Hum. Cell 1999, 12, 95–102. [Google Scholar] [PubMed]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Di, Y.-M.; Zhou, Z.-W.; Mo, S.-L.; Zhou, S.-F. Multidrug resistance-associated proteins and implications in drug development. Clin. Exp. Pharmacol. Physiol. 2010, 37, 115–120. [Google Scholar] [CrossRef]
- Liu, C.-M.; Kao, C.-L.; Tseng, Y.-T.; Lo, Y.-C.; Chen, C.-Y. Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell. Molecules 2017, 22, 1477. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lv, L.; Soroka, D.; Warin, R.F.; Parks, T.A.; Hu, Y.; Zhu, Y.; Chen, X.; Sang, S. Metabolism of [6]-Shogaol in Mice and in Cancer Cells. Drug Metab. Dispos. 2012, 40, 742–753. [Google Scholar] [CrossRef] [Green Version]
- Asami, A.; Shimada, T.; Mizuhara, Y.; Asano, T.; Takeda, S.; Aburada, T.; Miyamoto, K.; Aburada, M. Pharmacokinetics of [6]-shogaol, a pungent ingredient of Zingiber officinale Roscoe (Part I). J. Nat. Med. 2010, 64, 281–287. [Google Scholar] [CrossRef]
- Dong, Y.; Tu, J.; Zhou, Y.; Zhou, X.H.; Xu, B.; Zhu, S.Y. Silybum marianum oil attenuates oxidative stress and ameliorates mitochondrial dysfunction in mice treated with D-galactose. Pharmacogn. Mag. 2014, 10, 92–S99. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.; Zhong, H.; Tong, S.; Cao, X.; Firempong, C.K.; Liu, H.; Fu, M.; Yang, Y.; Feng, Y.; Zhang, H.; et al. Enhanced oral bioavailability of a sterol-loaded microemulsion formulation of Flammulina velutipes, a potential antitumor drug. Int. J. Nanomed. 2012, 7, 5067–5078. [Google Scholar]
- Hou, J.; Sun, E.; Sun, C.; Wang, J.; Yang, L.; Jia, X.B.; Zhang, Z.H. Improved oral bioavailability and anticancer efficacy on breast cancer of paclitaxel via Novel Soluplus((R))-Solutol((R)) HS15 binary mixed micelles system. Int. J. Pharm. 2016, 512, 186–193. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.-J.; Chen, X.-L.; Du, L.; Li, F. Quercetin-loaded freeze-dried nanomicelles: Improving absorption and anti-glioma efficiency in vitro and in vivo. J. Control. Release 2016, 235, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Q.; Sun, C.; Zhu, Y.; Yang, Q.; Wei, Q.; Chen, J.; Deng, W.; Adu-Frimpong, M.; Yu, J.; et al. Enhanced Oral Bioavailability, Anti-Tumor Activity and Hepatoprotective Effect of 6-Shogaol Loaded in a Type of Novel Micelles of Polyethylene Glycol and Linoleic Acid Conjugate. Pharmaceutics 2019, 11, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, L.H.; Morad, N.A.; Aan, G.J.; Makpol, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Mechanism of Chemoprevention against Colon Cancer Cells Using Combined Gelam Honey and Ginger Extract via mTOR and Wnt/beta-catenin Pathways. Asian Pac. J. Cancer Prev. 2015, 16, 6549–6556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vemuri, S.K.; Banala, R.R.; Subbaiah, G.P.V.; Srivastava, S.K.; Reddy, A.G.; Malarvili, T. Anti-cancer potential of a mix of natural extracts of turmeric, ginger and garlic: A cell-based study. Egypt. J. Basic Appl. Sci. 2017, 4, 332–344. [Google Scholar] [CrossRef]
- Yekta, Z.P.; Ebrahimi, S.M.; Hosseini, M.; Nasrabadi, A.N.; Sedighi, S.; Surmaghi, M.-H.S.; Madani, H. Ginger as a miracle against chemotherapy-induced vomiting. Iran. J. Nurs. Midwifery Res. 2012, 17, 325–329. [Google Scholar]
- Lete, I.; Allue, J. The Effectiveness of Ginger in the Prevention of Nausea and Vomiting during Pregnancy and Chemotherapy. Integr Med. Insights 2016, 11, 11–17. [Google Scholar] [CrossRef] [Green Version]
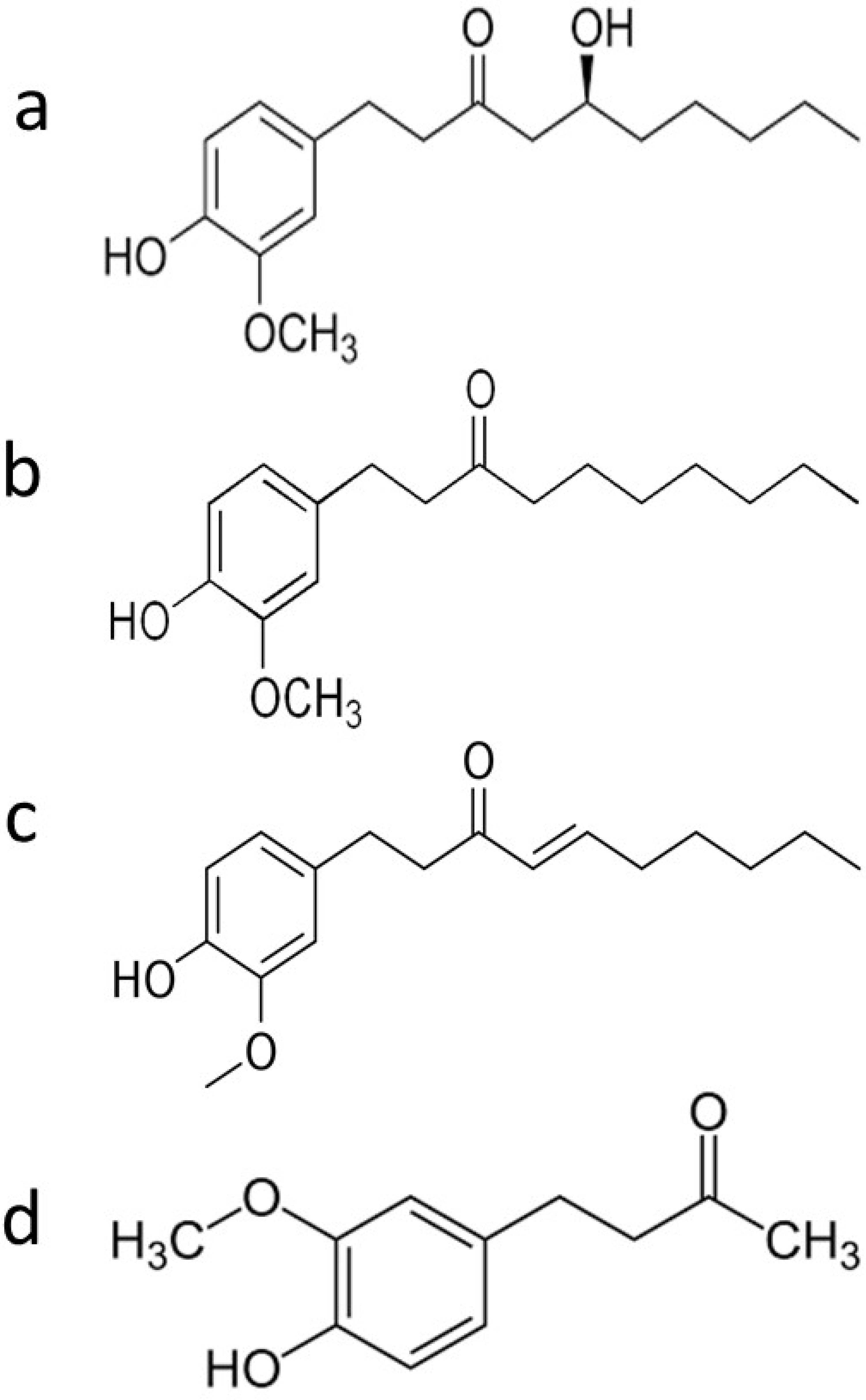


| Phenolic Ginger Compounds | Chemopreventive Activities | References |
|---|---|---|
| 6-gingerol | Blockage of the cell cycle at G2/M phase; decrease of cells in the SubG0 phase; depolarization and potential subsequent deterioration of the mitochondrial membrane; induction of apoptosis; inhibition of angiogenesis; induction of growth suppression; enhancement the doxorubicin efficacy | [8,9,10,11,12] |
| 6-paradol | Reduce blood glucose | [7] |
| 6-shogaol | Arrest of the cell cycle in G2/M phase; decrease levels of STAT3 and NF-κB-regulated target genes including cyclin D1; induce apoptosis; downregulation of surviving; decrease tumor volume and tumor burden; restore wild type p53 function; provoke autophagy; inhibit phase I enzymes (Cyt-p450 and Cyt-b5); increase phase II enzymes (GST, GR, and GSH); reduce the cleavage of Notch1 | [13,14,15,16,17] |
| Zingerone | Inhibition of TGF-β1 induced epithelial-mesenchymal transition, migration, and invasion | [18] |
| Tumor Entity | Functions of Ginger | References |
|---|---|---|
| Breast cancer | Blockage of the cell cycle at G2/M phase; Induction of typical apoptotic changes in nuclear morphology, chromatin condensation and fragmentation, membrane shrinkage and blebbing; enabled autophagy followed by caspase-independent apoptosis; induction of autophagy | [16,18,22] |
| Prostate cancer | Arrest of cell cycle in the G1 phase with subsequent decrease in S and G2/M through p21 dependent pathway; downregulation of MRP1 and GST-protein expression | [23,24] |
| Ovarian cancer | Suppressed production of NF-κB regulated angiogenic factors; p53 stimulation of apoptosis through Bcl-2 elimination | [25,26] |
| Colon cancer | Arrest of cell cycle at different check points by inhibition of cyclin dependent kinases and activation of cell cycle check points; upregulation of p21 expression; reverse of EMT to Mesenchymal–epithelial transition (MET) through the upregulation of miR-200c | [17,19,27,28] |
| Hepatocellular carcinoma | Arrest of cell cycle at the G2/M phase; inhibition of the PI3K/AKT/mTOR and STAT3 signaling pathways; inhibition of Bcl-2 expression and up-regulation of Bax, cytochrome c, caspase-9 and -3 protein expressions | [21,29] |
| Gastric adenocarcinoma | Interruption of cell cycle at different check points; mediation of mitochondrial pathway of apoptosis; unbalance ROS homeostasis and induction of apoptosis | [30] |
| Non-small lung epithelium cancer | The loss of mitochondrial membrane potential of that leads to increase in Bax/Bcl-2 ratio and activation of mitochondrial death cascade | [31,32] |
| Melanoma | Induction of caspase independent cell death via the inhibition of ERK1/2, p38 and Akt signaling pathway | [33] |
| Endometrial adenocarcinoma | Induction of apoptosis by increasing the expression of p53 and Bax and simultaneously decreasing the expression of Bcl-2 | [34] |
| Cervical cancer | Induction of typical apoptotic changes in nuclear morphology, chromatin condensation and fragmentation, membrane shrinkage and blebbing | [35] |
| Lung cancer | Sensitization of TRAIL-induced apoptosis by inhibiting autophagy flux | [36] |
| Head and neck squamous carcinoma | Increase in apoptotic death by downregulation of surviving; inhibition of mutant p53 Bcl-2 expression, and increased expression of Bax, regulation of Bax/Bcl-2 ratio which induce cell apoptosis | [37,38] |
| Pancreatic cancer | Activation of AMPK, a positive regulator of autophagy, and inhibition mTOR, a negative autophagic regulator; unbalance ROS homeostasis and induction of autosis | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadorozhna, M.; Mangieri, D. Mechanisms of Chemopreventive and Therapeutic Proprieties of Ginger Extracts in Cancer. Int. J. Mol. Sci. 2021, 22, 6599. https://doi.org/10.3390/ijms22126599
Zadorozhna M, Mangieri D. Mechanisms of Chemopreventive and Therapeutic Proprieties of Ginger Extracts in Cancer. International Journal of Molecular Sciences. 2021; 22(12):6599. https://doi.org/10.3390/ijms22126599
Chicago/Turabian StyleZadorozhna, Mariia, and Domenica Mangieri. 2021. "Mechanisms of Chemopreventive and Therapeutic Proprieties of Ginger Extracts in Cancer" International Journal of Molecular Sciences 22, no. 12: 6599. https://doi.org/10.3390/ijms22126599
APA StyleZadorozhna, M., & Mangieri, D. (2021). Mechanisms of Chemopreventive and Therapeutic Proprieties of Ginger Extracts in Cancer. International Journal of Molecular Sciences, 22(12), 6599. https://doi.org/10.3390/ijms22126599






